The National Agency for Food and Drug Administration and Control (NAFDAC) has warned Nigerians, especially health workers, about the fake Meronem 1g injection currently in circulation.
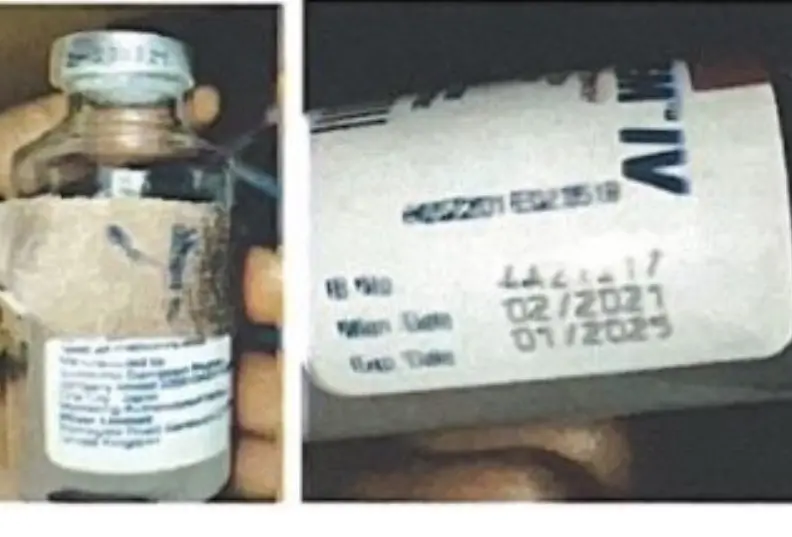
The head of the institution, professor. According to Pfizer’s Marketing Authorization Holder, Mojisola Adeyeye, an incident involving the suspicious purchase of Meronem 1g injection was reported through a patient reporting platform. He said the contents of the vial did not dissolve when it was dissolved for use by Pfizer and also noted an additional visual inspection of the packaging. It was also reported that the compression codes used for the 2A21F11 and 4A21I17 did not match the codes shown in the blank production batch documentation. Adeyeye said the manufacturing process did not meet Pfizer’s specifications. “The label on the bottle compares well with the designed version of the artwork. Meronem (meropenem trihydrate injection) is an antibiotic used to treat skin and abdominal (stomach area) infections caused by bacteria and meningitis (infection of the membranes around the brain and spinal cord) in adults and children older than 3 months. The introduction of counterfeit products poses a serious risk to patients as their quality and safety are not guaranteed,” Adeyemi said.
The NAFDAC boss added that health care providers and patients have been advised to purchase all health care products from approved or authorized suppliers. According to him, you should carefully check the authenticity and physical condition of the product before buying or entering it. Adeyeye urges importers, wholesalers and retailers to be vigilant in their supply chains and stop the illegal importation, distribution and sale of fake Meronem 1g injections and other substandard drugs.
He said anyone in possession of counterfeit products should stop using them and send them to the nearest NAFDAC office.






